Abstract
Patients (pts) with myelodysplastic syndromes (MDS) have few therapy options. Interventions to improve outcomes should consider strategies that arrest MDS in its early phases, when symptoms are minimal and prolonged survival is expected.
To develop prevention strategies that arrest MDS before the disease outcomes become irreversibly dismal, we dissected the molecular and biological mechanisms that maintain MDS in one of its premalignant phases, clonal cytopenia of undetermined significance (CCUS). Recognizing that CCUS is an aging-related disease, we first sought to determine, at the single-cell level, how CCUS affects the transcriptional and epigenetic profile of the aging hematopoietic stem and progenitor cell (HSPC) compartment and overcomes aging-induced degenerative phenotypes. We performed single-cell RNA sequencing (scRNA-seq) analysis of Lin -CD34 + HSPCs isolated from the bone marrow (BM) of 3 young healthy donors (yHDs), 4 elderly HDs (eHDs), and 3 elderly pts with CCUS carrying mutations in common MDS driver genes.
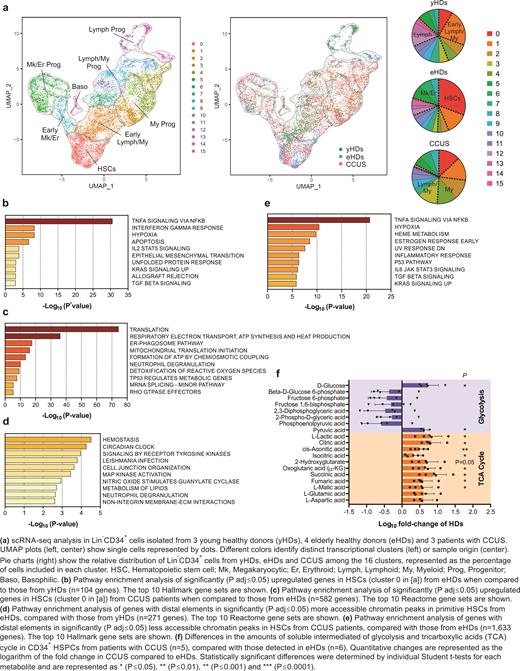
We found that the frequencies of hematopoietic stem cells (HSCs) and megakaryocytic (Mk)/erythroid (Er) progenitors were increased at the expense of myeloid (My) progenitors in eHDs as compared with yHDs (Fig. a). In contrast, CCUS pts had a predominant My-biased HSPC distribution (Fig. a). However, immunophenotypic quantification in large cohorts of eHDs and CCUS pts revealed that CCUS pts' BM had significantly fewer CD34 +CD38 - HSC populations and CD34 +CD38 + My progenitors, suggesting that My bias in CCUS results from the aberrant My differentiation of HSCs rather than My cell expansion. Further differential expression analysis among the scRNA-seq clusters showed that, compared with yHD HSCs, eHD HSCs were characterized by a significant upregulation of genes involved in the TNFα-induced activation of NF-κB (e.g., MCL1; Fig. b), which is consistent with previous findings that aged HSCs undergo transcriptional reprogramming to maintain their survival in response to changes in the systemic environment (He et al. Blood 2020). In contrast, CCUS HSCs, compared with eHD HSCs, overexpressed regulators of translation, respiratory electron transport, and mitochondrial translation initiation (Fig. c), which underscores these cells' state of proliferation and metabolic activation and their ability to overcome aging-induced phenotypic alterations.
To evaluate whether the aberrant lineage differentiation in eHD and CCUS HSPCs arose from the altered fate determination of HSCs, we performed single-cell assays for transposase-accessible chromatin sequencing to profile chromatin accessibility in sorted HSCs or Lin -CD34 + HSPCs from yHDs, eHDs, and CCUS pts. Consistent with our transcriptomic data, compared with yHD HSCs, eHD HSCs were mostly poised for Mk differentiation, whereas CCUS HSPCs were poised for lymphoid/My differentiation. Indeed, eHD HSCs had an increased activity of transcriptional factors belonging to the NF-κB family and open peaks at the distal elements of genes involved in hemostasis (Fig. d). In contrast, CCUS HSCs were poised to downregulate the expression of genes involved in NF-κB signaling and Mk/Er differentiation (Fig. e).
These results suggested that CCUS HSCs are highly metabolically active to maintain My differentiation. Indeed, metabolomic analyses confirmed that intermediates of oxidative phosphorylation were significantly upregulated in CCUS CD34 + cells as compared with eHD CD34 + cells (Fig. f). Further, scRNA-seq analysis of mononuclear cells isolated from the BM of 3 CCUS and 3 eHD samples revealed the widespread upregulation of genes involved in protein processing and mitochondrial metabolism. This analysis also revealed impaired terminal My differentiation despite the HSPC My bias, with decreased frequencies of monocytic cells, and an intriguing expansion of cytotoxic cell subsets in the BM of CCUS pts.
In conclusion, our results demonstrate that CCUS HSCs carrying MDS driver mutations evade aging-induced phenotypic degeneration, become metabolically active, and have aberrant My skewing. Our study clarifies the molecular mechanisms underlying MDS initiation and offers an opportunity for early therapeutic intervention.
No relevant conflicts of interest to declare.